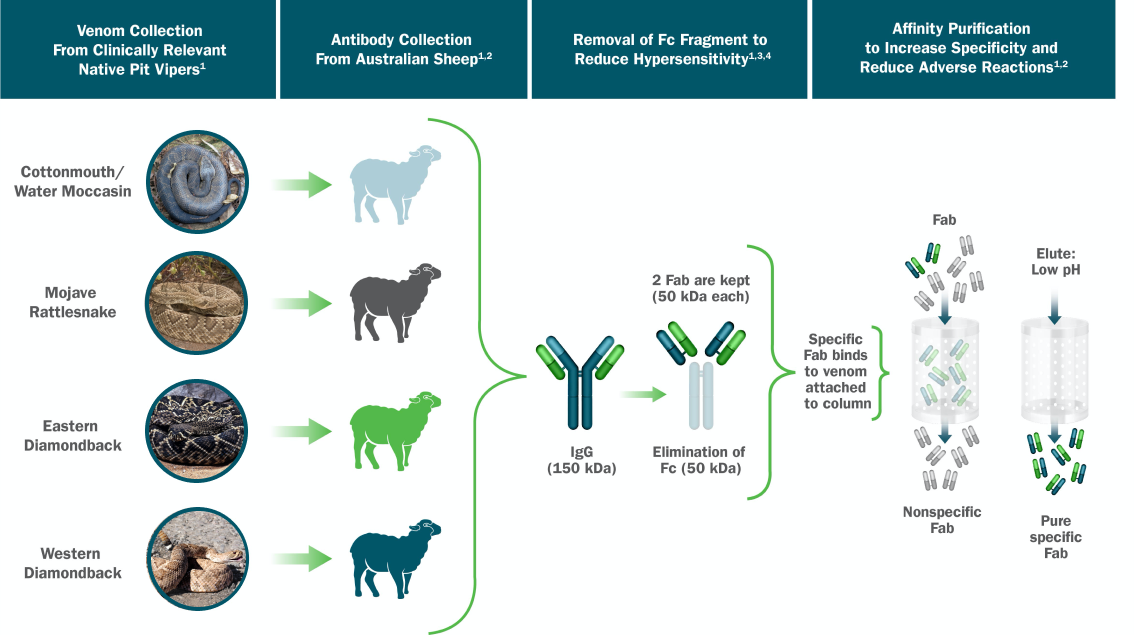
Cottonmouth/
Water Moccasin

Eastern
Diamondback

Mojave
Rattlesnake

Western
Diamondback